

Electron Volt -> cubic yard of atmosphere.Random ENERGY units gallon atmosphere UK thermie british thermal unit IT horsepower hour nanojoules megagrams of TNT exaelectron volt yottawatt hour gigawatt hour newton meter (Electron Volt) to (Ergs) conversions = 0.036749405469679 atomic unit of energy (au) Science Physics How many volts of electrical potential is 324 ergs per elementary charge How many volts of electrical potential is 324 ergs per elementary charge Physics for Scientists and Engineers: Foundations and Connections 1st Edition ISBN: 9781133939146 Author: Katz, Debora M. The electron-volt (eV) is a unit of energy (the energy an electron would gain after acceleration through a potential of 1 volt). In relation to the base unit of => (joules), 1 Electron Volt (eV) is equal to 1.60218E-19 joules, while 1 Ergs (ergs) = 1.0E-7 joules.
#Charge of electron in ergs how to#
How to convert Electron Volt to Ergs (eV to ergs)?Īlways check the results rounding errors may occur.
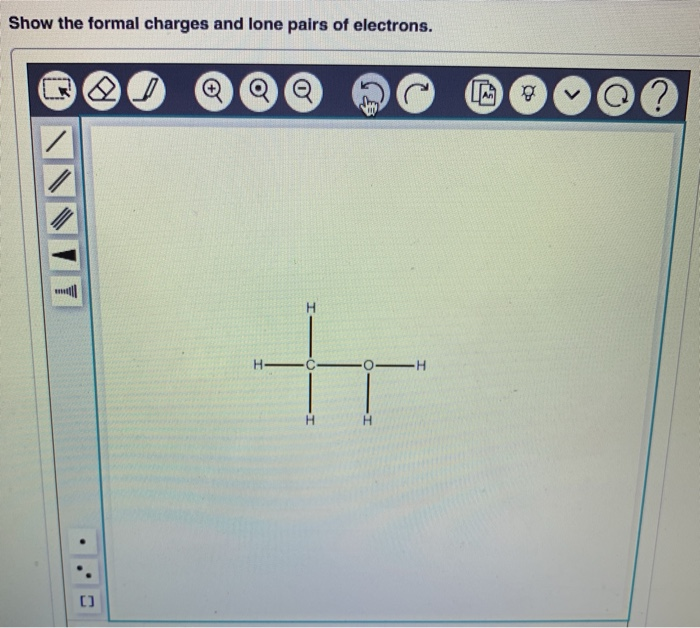
We get q = - 1 e = - 1.6 x 10-19 Coulomb from the equation q = ne (with n = 1).The base unit for energy is joules (Non-SI/Derived Unit) the possible charge of electron value for one electron is. It is thus equal to 10 7 joules or 100 nanojoules ( nJ) in SI units. Potential, statvolt, 299.8 Magnetic Field, Gauss, 10 Further units: 1 eV 1.602.10-12 erg 1 Ry 13.6 eV (ionization energy of. In the CGS base units, it is equal to one gram centimetre-squared per second -squared (gcm 2 /s 2 ). If a body has n1 electrons and n2 protons, then the total charge of electron of the body is:īecause n1 and n2 are both integers, their difference must be an integer as well.Īny charge of electrons body can have a charge of electron of +/- e, +/- 2e, +/- 3e, +/- 4e, and so on, i.e. An erg is the amount of work done by a force of one dyne exerted for a distance of one centimetre. e, Elementary charge of an electron, 4.803250(21) x 10-10 ESU m Rest mass of the electron, 9.109558(54) x 10-28 g G, Gravitational constant, 6.6732(31) x 10. The resulting emission is called freefree radiation. Hence it can be used the express the charge possessed by any body, not necessarily a proton or electron. In physics, this is one of the fundamental constants. where me9.1×10-28 g is the electron mass and r is the distance between the electron and the ion. Gives the charge of electron q of a body.Īs a result, the charge of electron on an electron is (e -) and the charge of proton is (+e).Į = 1.6 x 10-19 coulomb or C is the value of the basic unit of elementary charge of electron. All atoms have the same number of electrons as protons, so the positive and negative charges 'cancel out', making atoms electrically neutral. If m and e are the mass and charge of the revolving electron in the orbit of radius r for hydrogen atom, the total energy of the revolving electron will be.
#Charge of electron in ergs free#
The property of all free charge of electrons being an intrinsic element of a basic unit of charge of electron represented by e is known as the quantization of electric charge. Electrons have an electric charge of 1, which is equal but opposite to the charge of a proton, which is 1+. The mass of of an electron is measured in electron volts (eV) and corresponds to a rest energy of Mev = 0.511 eV. So we have negative 'e', is the charge on the electron, divided by 'r squared', is equal to the mass of the electron times the centripetal.


 0 kommentar(er)
0 kommentar(er)
